Coupling Endergonic and Exergonic Reactions
Enter the email address you signed up with and well email you a reset link. The addition of the 4-aminopyridine-boryl radical to 1a is exergonic to give a.
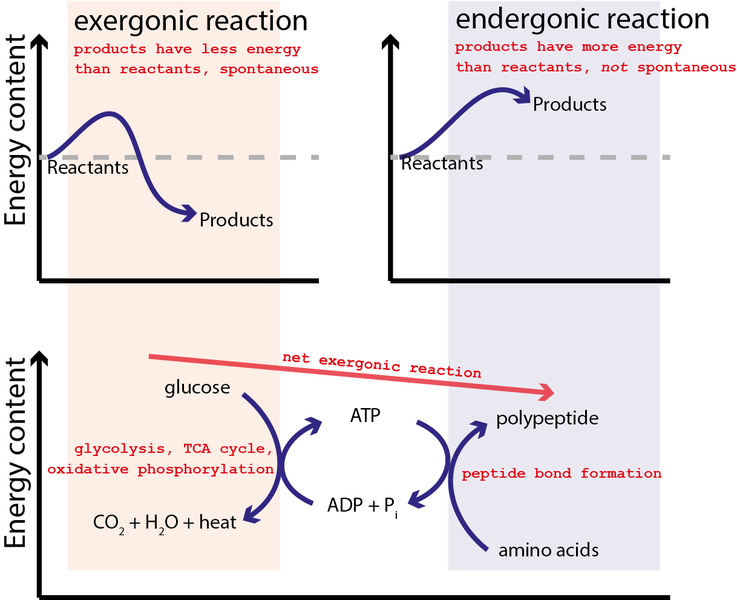
9 6 Coupled Reactions Chemistry Libretexts
However the free-energy change for these elementary reactions involving charge transfer will become negative that is exergonic reaction when the electrode potential U is lower than a limiting.

. ΔG 239 kcalmol and is endergonic by 121 kcalmol fig. The products have weaker bonds than the reactants. Adenosine triphosphate ATP is the intermediate molecule that drives the exergonic transfer of energy to switch to endergonic anabolic reactions used in muscle.
Endergonic exergonic exothermic and endothermic. Also within the scope of bacterial metabolism is the study of the uptake and utilization of the inorganic or organic compounds required for growth and maintenance of a cellular steady state assimilation reactions. It is the opposite of an exergonic reaction.
A quantitative measure of the favorability of a given reaction under these conditions is the change ΔG sometimes written delta G or dG in Gibbs. The products lend themselves to rapid derivatization Scheme 2BC. Causing the exergonic form to release high quantities of energy.
In biochemistry a metabolic pathway is a linked series of chemical reactions occurring within a cellThe reactants products and intermediates of an enzymatic reaction are known as metabolites which are modified by a sequence of chemical reactions catalyzed by enzymes. Here we present a universal design principle to evaluate the activity. 26 In most cases of a metabolic pathway the product of one enzyme acts as the substrate for the.
Metabolism is the total of all catabolic exergonic anabolic endergonic reactions. Also called a heat absorbing nonspontaneous reaction or an unfavorable reaction is a chemical reaction in which the standard change in free energy is positive and an additional driving force is needed to perform this reaction. The calculations also suggested that complexation of NaI and PPh 3 is exergonic in acetonitrile through the cation-π interaction exergonic by 46 kcalmol.
Gibbs free energy and spontaneous reactions. Thus this route is considered less feasible. According to the second law of thermodynamics for systems reacting at fixed temperature and pressure without input of non-Pressure Volume PV work there is a general natural tendency to achieve a minimum of the Gibbs free energy.
In order to transform this energy into an endergonic form the coupled response plays an essential role. MS-PCETs are redox mechanisms in which both an electron and a proton are exchanged together often in a concerted elementary step. The ironsulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser a Munich patent lawyer with a degree in chemistry who had been encouraged and supported by philosopher Karl R.
The energy to phosphorylate ADP comes from catabolic reactions in the cell The ATP cycle is a revolving door through which energy passes during its transfer from catabolic to anabolic pathways Energy from catabolism exergonic energy-releasing processes Energy for cellular work endergonic energy-consuming processes ATP ADP P i H. Thus endergonic reactions are thermodynamically unfavorable. Popper to publish his ideas.
Adenosine triphosphate or ATP is often called the energy currency of the cell because this molecule plays a key role in metabolism particularly in energy transfer within cells. The hypothesis proposes that early life may. In the second reaction the formation of the bond between the carboxylic group at C-1 of 13-bisphosphoglycerate and orthophosphate occurs to form an anhydride called acyl phosphate.
This further underlines the unique character and orthogonality of the coupling reaction presented herein. The assembly of NaI and PPh 3 with the RAE N -cyclohexanecarbonylphthalimide to form a charge transfer complex CTC via coulombic interaction is calculated to be exergonic by 38 kcal. It has a positive ΔG because it takes more energy to break the bonds of the reactant than the energy of the products offer ie.
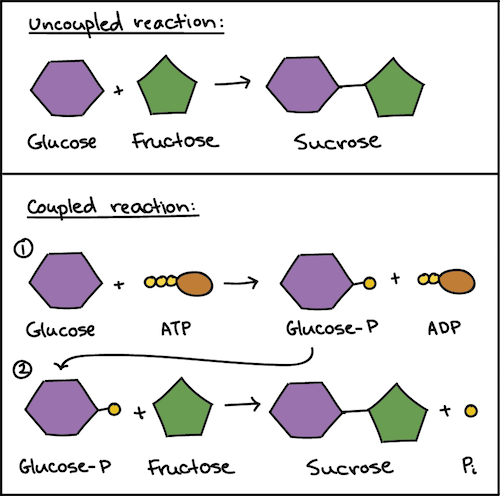
ATP and reaction coupling. However it should be noted that ATP participates in the phosphorylation reaction whereas the hydrolytic cleavage of the phosphate group releases energy in. This process allows endergonic reactions to be driven by exergonic ones and is an alternative less recognised mechanism of energy coupling to the well-known chemiosmotic principle.
The reaction is quite endergonic with a G of 493 kJmol 118 kcalmol. Biology is brought to. The observed chalcogenide exchange illustrates that this Fe 4 S 4 cluster is capable of core substitution reactions under certain.
Mannich reactions the desired β-amino amide 4 was not detected. The reaction is quite exergonic with a G of -43 kJmol -103 kcalmol. The chain of redox reactions driving the flow of electrons through the electron transport chain from electron donors such as NADH to electron acceptors such as oxygen and hydrogen protons is an exergonic process it releases energy whereas the synthesis of ATP is an endergonic process which requires.
Endergonic exergonic exothermic and endothermic. CONCEPT 83âATP powers cellular work by coupling exergonic reactions to endergonic reactionsâ CONCEPT 84âEnzymes speed up metabolic reactions by lowering energy barriersâ CONCEPT 85âRegulation of enzyme activity helps control metabolismâ 9 Cellular Respiration and Fermentationâ Life Is Workâ. Endergonic reactions require energy and include anabolic reactions and the contraction of muscle.
The carbon on Earth simply moves between different reservoirs because of geological and geochemical processes as well as human activities while the total carbon amount has remained constant The new technology for CO 2 utilization should at least be carbon-neutral either via exergonic pathways or endergonic ones but driven by renewable energy. The molecule acts to couple the energy of exergonic and endergonic processes making energetically unfavorable chemical reactions able to proceed. In chemical thermodynamics an endergonic reaction from Greek ἔνδον endon within and ἔργον ergon work.
We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer MS-PCET in organic synthesis. Additionally endergonic reactions are usually anabolic. These respective exergonic energy-yielding and endergonic energy-requiring reactions are catalyzed within the living bacterial.
As such MS-PCET can function as a non-classical mechanism for. Enthalpy Which of the following statements regarding enzymes is true. Developing highly active single-atom catalysts for electrochemical reactions is a key to future renewable energy technology.
Hetero-coupling reactions also known as cross-coupling reactions. Dephosphorylation reactions catalysed by fructose-bisphosphatase and glucose-6-phosphatase provide exergonic reactions which reverse exergonic glycolytic reactions by a different mechanism. In laymans terms the total amount.
Although several examples of coupling reactions between α-bromo- and α-iododifluoroacetamides with alkenes have recently been reported. The electricity then is not extracted by the machine as heat. Study with Quizlet and memorize flashcards containing terms like Choose the pair of terms that correctly completes this sentenceCatabolism is to anabolism as ____ is to ____.
Cleavage of 3o bearing an indoline amide can easily be achieved through oxidative conversion to the.
How Are Endergonic And Exergonic Reactions Related In Terms Of The Role Of Atp In Cells Quora
Atp Cycle And Reaction Coupling Energy Article Khan Academy

What Atp Is And Why It S Important In Metabolism Chemistry Molecules Metabolism

No comments for "Coupling Endergonic and Exergonic Reactions"
Post a Comment